The most effective drug for Alzheimer’s will be blocked for use on the NHS on Wednesday.
Regulators are expected to declare the new treatment for the disease safe for use but the rationing body for the health service will immediately rule that it is too expensive for NHS patients.
The decision is set to disappoint charities and campaigners who have called for better access to newly emerging drugs on the NHS.
Donanemab has been described as game-changing and research showed it slowed the progression of Alzheimer’s by 35 per cent. Scientists said it could mean patients are able to live at home with a better quality of life for an extra two years.
The decision on donanemab – the second drug found to slow the progress of Alzheimer’s disease – is set to mirror one taken in August when lecanemab, the first breakthrough treatment for the condition, was licensed.
The draft guidance from the Medicines and Healthcare products Regulatory Agency (MHRA) will mean patients will only be able to obtain either drug from private clinics unless they are part of clinical trials. Health insurance policies are unlikely to cover costs.
Charities and pharmaceutical companies have criticised the National Institute for Health and Care Excellence (Nice) – the rationing body for the NHS – for disregarding the costs borne by families and society, in making their assessments.
Almost 1 million people in the UK are living with dementia, including one in six people over the age of 80. The figure is expected to reach 1.4 million by 2040 as Britain ages.


The vast majority of care is either provided by loved ones or paid for privately, but Nice excludes these “non-medical” costs of care in their decision making.
Estimates suggest that the UK spends approximately £42 billion a year on dementia, with most of the costs borne by families and social care bodies. Forecasts have said that this figure could reach £90 billion by 2040.
The MHRA’s decision has been hit by delays, with the regulator initially planning to make a call in July – the same time that it was approved for use in the US.
Prof Sir John Hardy, the chairman of molecular biology of neurological disease at the UCL Institute of Neurology and one of the world’s leading researchers in the field, said he expected Nice to once again “come down on the wrong side of the argument” about drugs which were “game-changing”.
The scientist was the first to identify the role of amyloid in Alzheimer’s disease which has now led to drugs which work by clearing the protein.

Prof Hardy said: “These drugs can give people an extra two years at home, rather than in a nursing home. That is time enjoying their lives, having holidays, this is important stuff.”
The scientist – who made clear that he has consulted for both Eisai – which manufactures lecanemab – and Lilly – which makes donanemab – said: “These are finely balanced arguments, but I do think they’ve come down on the wrong side of it.”
“I also think that the benefit of approval would be that it would kick NHS dementia care into shape – which really needs to happen. These drugs will come down the line at some point, and I don’t think the NHS is ready for them,” he said.
Lilly estimates the cost of the drug is $32,000 (£24,600) in the US, which is about 25 per cent higher than lecanemab.
But it has the advantage of being a 30-minute monthly intravenous injection, instead of an hour every two weeks, slashing the cost of administering it for the NHS.
In the US, total treatment costs for donanemab – including monitoring and scans – average $78,000 per year (£60,000) per patient.
Patients can also stop taking it if it adequately clears the amyloid protein in the brain that the drug targets.
By contrast, lecanemab is given indefinitely, until disease reaches a moderate stage.
In trials, donenamab slowed cognitive decline by 35 per cent, making it even more effective than lecanemab, which slowed the disease by 27 per cent.
Almost half of participants on donanemab had no clinical progression of disease after a year, compared with 29 per cent on placebo.
However, it has a higher risk of side effects, with the proportion of patients suffering brain bleeds or swelling twice as high as its competitor.
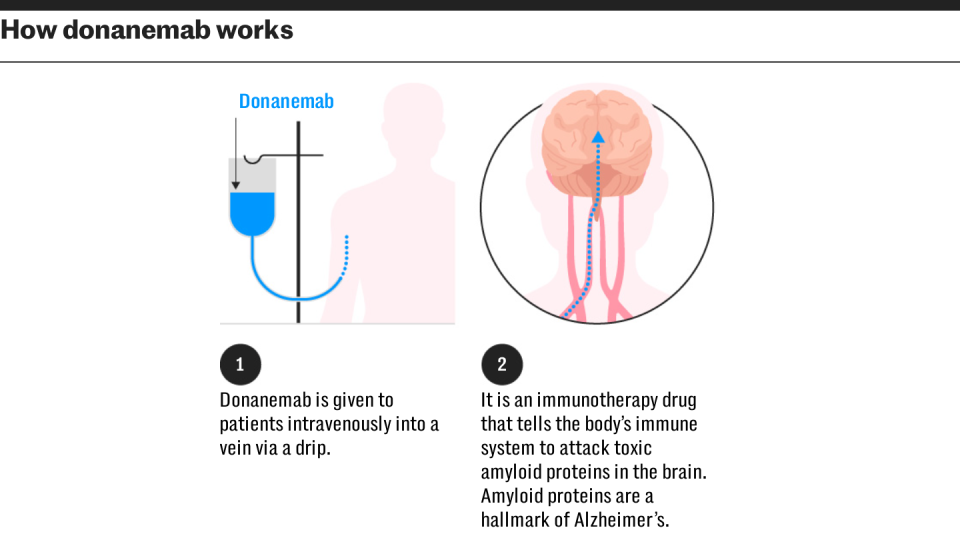
Both drugs work in a similar way and are a form of monoclonal antibody, replicating the action of the immune system to target specific proteins, in this case the removal of amyloid from the brain.
Charities and pharmaceutical companies have said that patients will miss out on the best new treatments if the NHS does not fundamentally change the way it calculates the value of drugs.
Charity Alzheimer’s Research UK wrote to Wes Streeting, the Health Secretary, in August and said his leadership was “needed to help enable fast and equitable access to a new generation of treatments”.
Chris Stokes, UK general manager of Eli Lilly and Company, which makes donanemab, this week urged Nice to consider a treatment’s economic and social benefits as well as how many “quality years” it can add to a patient’s lifespan.
These include whether taking a drug could make it easier for someone to get back to work, or reduce their need for carers.
“If we don’t incorporate the wider value into the discussion, then there is a real risk that patients will miss out on innovative treatments,” Mr Stokes told The Sunday Times.
When lecanemab was rejected in Nice’s draft guidance, the Alzheimer’s Society said families across the country had been left in “uncertainty and confusion”.
Alzheimer’s Research UK said the failure to include the cost of caring in the model was “fundamentally unjust” saying “the way these assessments are being carried out is just not fit for purpose”.
Broaden your horizons with award-winning British journalism. Try The Telegraph free for 3 months with unlimited access to our award-winning website, exclusive app, money-saving offers and more.
